Scientists May Have Found The Key Cellular Process Behind Aging in Animals : ScienceAlert
Groundbreaking Study Reveals How Cells “Remodel” Their Largest Organelle During Aging
In a discovery that could revolutionize our understanding of aging and potentially unlock new treatments for age-related diseases, researchers from Vanderbilt University School of Medicine have uncovered a remarkable cellular adaptation that occurs as we grow older.
The study, published in Nature Cell Biology, reveals that as organisms age, their cells actively reorganize one of the most critical structures inside them: the endoplasmic reticulum (ER). This extensive network, which extends throughout the cell like a complex highway system, plays essential roles in protein folding, lipid synthesis, and cellular organization.
The Cellular Factory Gets a Makeover
“Think of it like a factory reorganizing its layout to meet changing production demands,” explains Kris Burkewitz, the study’s lead biologist. “When space is limited or production needs change, the factory has to adapt its configuration to continue making the right products efficiently.”
What makes this discovery particularly fascinating is that this remodeling isn’t random damage—it’s an active, protective response by cells to the challenges of aging.
The Endoplasmic Reticulum: Cell’s Unsung Hero
The endoplasmic reticulum is one of the largest and most complex structures in our cells. It comes in two main varieties:
- Rough ER: Studded with ribosomes, it’s responsible for protein synthesis, folding, and transport
- Smooth ER: Handles lipid synthesis, calcium storage, and detoxification
This cellular powerhouse doesn’t just perform biochemical tasks—it also acts as a scaffold, helping organize other cellular components and maintaining the cell’s internal architecture.
What the Researchers Discovered
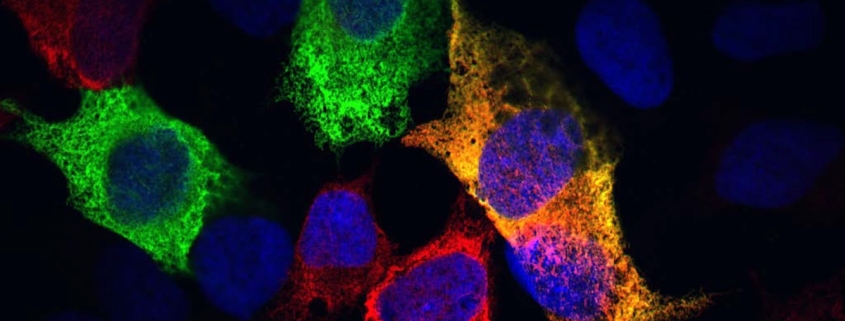
Using Caenorhabditis elegans nematodes—transparent worms that age rapidly and are ideal for cellular observation—the research team employed advanced fluorescence and electron microscopy to watch cells age in real-time.
The findings were striking: as the worms aged, the amount of rough ER in their cells dramatically decreased, while the smooth ER remained relatively stable. This selective reduction suggests cells are actively prioritizing certain functions over others as they age.
Why This Matters for Human Health
This discovery has profound implications for human health and longevity. While medical advances have extended human lifespans dramatically—with some individuals living well beyond 100 years—these extra years don’t always come with good health. Many elderly people spend their later years battling chronic diseases and declining function.
The research suggests that ER remodeling might be one of the earliest triggers in the aging cascade, potentially setting off the chain of events that leads to cellular dysfunction and disease.
The Mechanism Behind the Magic
The cells accomplish this remodeling through a process called ER-phagy, a specialized form of autophagy (the cell’s recycling system). In ER-phagy, cells selectively target specific portions of the endoplasmic reticulum—particularly damaged or excess sections that could threaten cellular health—for breakdown and recycling.
This isn’t just damage control; the study indicates this is a proactive strategy cells employ to maintain function as they age.
Looking Forward: Potential Drug Targets
The most exciting aspect of this discovery is its potential for therapeutic intervention. If scientists can understand exactly how cells control this remodeling process, they might be able to develop drugs that:
- Enhance the protective aspects of ER remodeling
- Prevent harmful changes that lead to disease
- Promote healthy cellular aging even in older organisms
“This could be a game-changer for treating age-related chronic diseases,” Burkewitz notes. “Changes in the ER occur relatively early in the aging process, making it an attractive target for intervention before more serious problems develop.”
The Bigger Picture
This research adds to a growing body of evidence that aging isn’t simply the accumulation of random damage, but rather involves coordinated cellular responses—some protective, others potentially harmful. Understanding these responses could be the key to not just living longer, but living better.
The Vanderbilt team plans to continue investigating ER dynamics throughout the aging process, aiming to clarify exactly what drives these changes and how they might be manipulated to promote healthy longevity.
As we continue to push the boundaries of human lifespan, discoveries like this remind us that the secret to healthy aging might lie not in preventing all cellular changes, but in understanding and guiding the ones that nature has already programmed into our cells.
Tags: #AgingResearch #CellBiology #EndoplasmicReticulum #Longevity #MedicalBreakthrough #HealthyAging #CellularRemodeling #ERphagy #VanderbiltUniversity #NatureCellBiology
Viral Sentences:
- Scientists discover cells actively “remodel” their largest organelle during aging
- Groundbreaking study reveals aging is a coordinated cellular strategy, not just damage
- Endoplasmic reticulum remodeling could be the key to treating age-related diseases
- Researchers watch cells age in real-time using transparent worms
- The factory analogy: how cells reorganize their internal machinery to survive longer
- Medical science extends lifespans, but can we now extend healthspans too?
- Discovery of protective cellular response that happens early in aging process
- Potential drug target identified for preventing age-related chronic diseases
- Rough ER dramatically decreases with age while smooth ER stays stable
- Autophagy’s secret weapon: ER-phagy and cellular recycling
- Transparent nematodes become windows into human aging
- Could controlling ER remodeling help us all live healthier longer?
,


Leave a Reply
Want to join the discussion?Feel free to contribute!