WHO slams US-funded newborn vaccine trial as “unethical”
Controversial Hepatitis B Vaccine Trial in Guinea-Bissau Suspended Amid Global Ethical Concerns
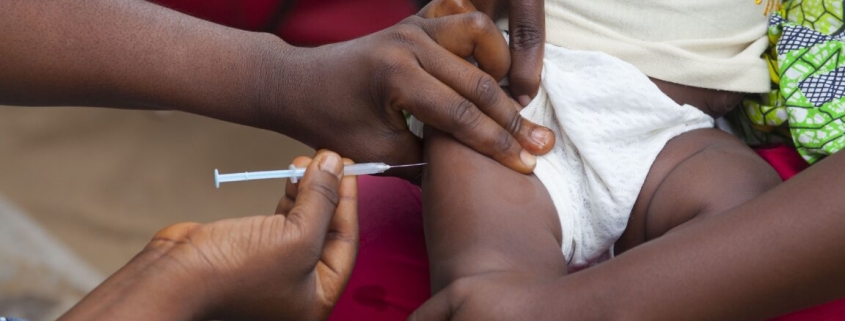
A controversial clinical trial testing the hepatitis B vaccine in newborns has been suspended in Guinea-Bissau following mounting international pressure and sharp criticism from the World Health Organization (WHO). The trial, which aimed to study the effects of withholding the hepatitis B birth dose vaccine, has sparked a fierce debate over medical ethics, global health policy, and the rights of vulnerable populations.
The WHO issued a strongly worded statement condemning the trial, emphasizing that “exploiting scarcity is not ethical.” The agency underscored the critical importance of the hepatitis B vaccine birth dose, describing it as “an effective, and essential public health intervention” that has been used for over three decades in more than 115 countries. The vaccine is designed to prevent life-threatening liver disease by stopping mother-to-child transmission at birth, a particularly pressing issue in Guinea-Bissau, where over 12 percent of adults suffer from chronic hepatitis B.
The Ethical Dilemma
The WHO’s statement laid out a series of ethical concerns regarding the trial’s design and execution. The agency argued that the trial protocol does not appear to ensure even a minimum level of harm reduction and benefit to study participants. Specifically, the WHO pointed out that the trial does not include screening pregnant women or vaccinating newborns exposed to hepatitis B, leaving them vulnerable to serious and potentially irreversible harm, including chronic infection, cirrhosis, and liver cancer.
The WHO also highlighted the lack of scientific justification for withholding a proven intervention. There is no credible evidence of the safety concerns that the trial’s proponents claim to be investigating. Furthermore, the trial’s single-blind, no-treatment-controlled design raises significant concerns about bias, potentially limiting the interpretability and policy relevance of the results.
Trial Suspension and International Response
As of now, the trial appears to be suspended. According to Nature News, health officials in Guinea-Bissau announced during a January 22 press conference that a technical and ethical review was pending. Quinhin Nantote, the Minister of Public Health for Guinea-Bissau, stated, “There has been no sufficient coordination in order to take a final decision regarding the study. Faced with this situation, we decided to suspend it.”
The Africa Centres for Disease Control and Prevention had previously suggested that the trial would not proceed. However, the U.S. Department of Health and Human Services issued a statement indicating that the trial was “proceeding as planned,” adding to the confusion and controversy surrounding the issue.
Global Implications
The suspension of the trial has far-reaching implications for global health policy and medical research ethics. It underscores the importance of adhering to established ethical standards in clinical trials, particularly when dealing with vulnerable populations. The WHO’s intervention serves as a reminder that the pursuit of scientific knowledge must never come at the expense of human rights and well-being.
The controversy also highlights the need for greater transparency and accountability in international research collaborations. As the global community continues to grapple with complex health challenges, it is essential that all stakeholders—governments, research institutions, and international organizations—work together to ensure that ethical principles are upheld and that the benefits of medical advancements are equitably distributed.
Conclusion
The suspension of the hepatitis B vaccine trial in Guinea-Bissau is a significant development in the ongoing debate over medical ethics and global health policy. While the trial’s proponents argue that it could provide valuable insights into vaccine safety and efficacy, the WHO and other critics contend that the potential risks to participants far outweigh any potential benefits. As the review process continues, the international community will be watching closely to see how this case unfolds and what lessons can be learned to prevent similar ethical breaches in the future.
In the meantime, the WHO’s statement serves as a powerful reminder of the importance of prioritizing the health and well-being of all individuals, particularly those in vulnerable communities. As we move forward, it is crucial that we continue to uphold the highest ethical standards in medical research and ensure that the pursuit of scientific knowledge is always guided by a commitment to human dignity and justice.
Tags: Hepatitis B vaccine, Guinea-Bissau, WHO, medical ethics, clinical trials, global health, vaccine safety, mother-to-child transmission, liver disease, chronic infection, cirrhosis, liver cancer, ethical standards, vulnerable populations, international research, health policy.
Viral Phrases: “Exploiting scarcity is not ethical,” “life-threatening liver disease,” “serious and potentially irreversible harm,” “no scientific justification,” “substantial risk of bias,” “technical and ethical review,” “suspended trial,” “global health policy,” “medical research ethics,” “human rights and well-being.”
,


Leave a Reply
Want to join the discussion?Feel free to contribute!